| PREVIOUS PRESENTATION | BACK TO PROGRAM OVERVIEW | NEXT PRESENTATION |
Detection of Lung Cancer Cells by A Voltage-controlled Terahertz Chemical Microscopy
Xue Ding1, Mana Murakami1, Jin Wang1, Hirofumi Inoue2, Toshihiko Kiwa1
1Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama, Japan
2Graduate School of Medicine Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan
Since 1981, cancer has been the first largest mortality rate in Japan and reaches nearly 30 percent of all deaths. Cancer genome analysis has recently attracted attention for personalized cancer treatment and provide the best treatment for patients through precise analysis of the genome. In this treatment, the accuracy of cancer genome analysis depends on evaluation of the ratio of cancer cells in a specimen tissue. Conventionally, specimen tissues were fixed by a formalin-fixed paraffin-embedded (FFPE), and the ratio of cancer cells and normal cells were visually evaluated using a microscope. However, this fixing protocol takes at least two days, and visual evaluation depends on the pathologists’ skill. So far, we have developed a terahertz microscope and successfully detected cancer cells in solutions [1]. Recently, we have proposed a new method to improve the sensitivity of lung cancer cell detection by applying voltage to the sensing plate [2].
A terahertz chemical microscope (TCM) is one of an excellent option to detect antigens in a very small volume of solutions [3-5]. The TCM used a sensing plate, as shown in Figure 1(a), which consist of SiO2 film and Si film on a sapphire substrate. When the femtosecond laser irradiates the sensing plate from the substrate side, THz pulses are generated and radiated to free space by a surface field effect of the Si film. Generally, an antibodies are immobilized on the surface of the sensing plate to detect a specific antigen. Because the surface field changes by change of the chemical potentials of the surface of the sensing plate due to the conjugation of antigens and immobilized antibodies, the amplitude of the THz pulses are also change. Thus, the TCM can detect the conjugation by measuring the amplitude of the radiated THz pulses. In this study, biotin-labeled cytokeratin AE1/AE3 (Protein A or G purified, Novus Biologicals, Briarwood Avenue, Centennial, CO, USA) was used as an antibody that immobilized on the sensing plate using avidin-biotin reaction. After that, lung cancer cells(PC9) reacted with immobilized antibodies. PC9 can be detected by detecting changes in terahertz wave intensity before and after the reaction. However, since the terahertz wave intensity is proportional to the square root of the chemical potentials, the chemical potentials on the surface of the sensing plate is too high and the terahertz amplitude intensity will reach saturation. Therefore, in this study, as shown in Figure 1(b) we attempted to control the region of terahertz radiation intensity saturates by applying an offset voltage to the Si layer of the sensing plate to optimize the free space before the reaction of biologically relevant substances.
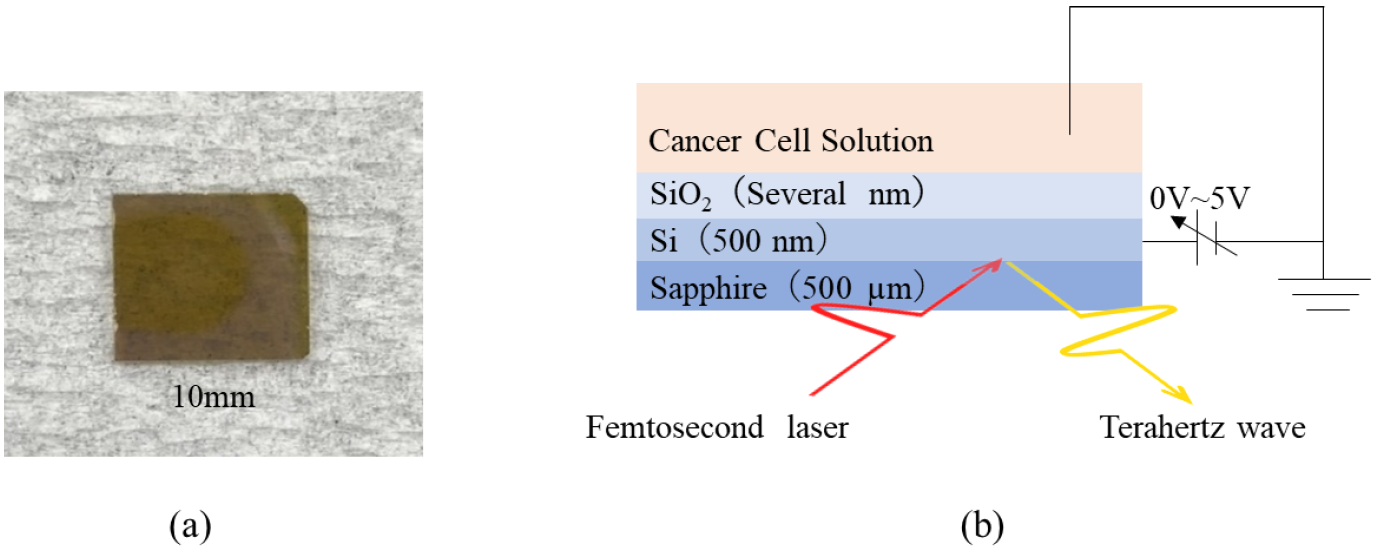
Figure 1: (a) Physical picture of the sensing plate. (b) Conceptual picture of sensing plate voltage application circuit.
Figure 2 (a) shows the change in amplitude of THz pulses as a function of the of concertation of lung cancer cells (PC9) before and after the reaction with cytokeratin AE1/AE3 antibody (purified protein A or G, Novus Biologicals, Briarwood Avenue, Centennial, CO, USA) at different offset voltages. Figure 2 (b) shows the sensitivity of the sensors obtained by liner-fit of the plots shown in Fig.2(a). The sensitivity was 0.705 ± 0.07217 mV/dec. and 2.025 ± 0.04907 mV/dec. for applied voltages of 0 V and 2 V, respectively. The results show that the applied voltage can change the sensitivity of the detection of cancer cells.
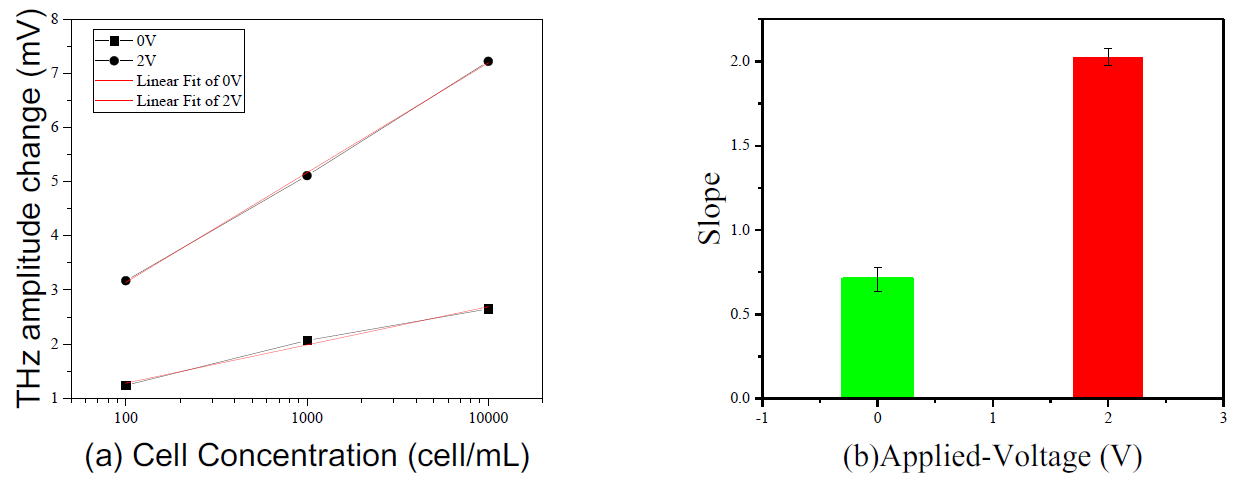
Figure 2: (a) The THz amplitude changes as a function of the log number of PC9 cells at 0V and 2V offset voltages. (b) is the slope of the linear fit of 0V and 2V.
References
[1] Y. Yoshida, X. Ding, K. Iwatsuki, K. Taniizumi, H. Inoue, J. Wang, K. Sakai, T. Kiwa, “Detection of Lung Cancer Cells in Solutions Using a Terahertz Chemical Microscope,” Sensors 21, 7631 (2021)
[2] X. Ding, M. Murakami, J. Wang, H. Inoue, T. Kiwa, “Sensitivity Improvement of Lung Cancer Cells Detection Using Voltage-controlled Terahertz Chemical Microscope,” submitted to CREO-PR2024, Korea.
[3] T. Kiwa, J. Kondo, S. Oka, I. Kawayama, H. Yamada, M. Tonouchi, and K. Tsukada, “Chemical sensing plate with a laser-terahertz monitoring system,” Applied Optics 47(18) 3324-3327 (2008)
[4] T. Kiwa, Y. Kondo, Y. Minami, I. Kawayama, M. Tonouchi, K. Tsukada, “Terahertz chemical microscope for label-free detection of protein complex,” Applied Physics Letters 96(21), 211114 (2010)
[5] T. Kiwa, T. Kamiya, T. Morimoto, K. Sakai, K. Tsukada, “pH measurements in 16-nL-volume solutions using terahertz chemical microscopy,” Optics Express 26(7), 8232-8238 (2018)
